Biochemical Assays
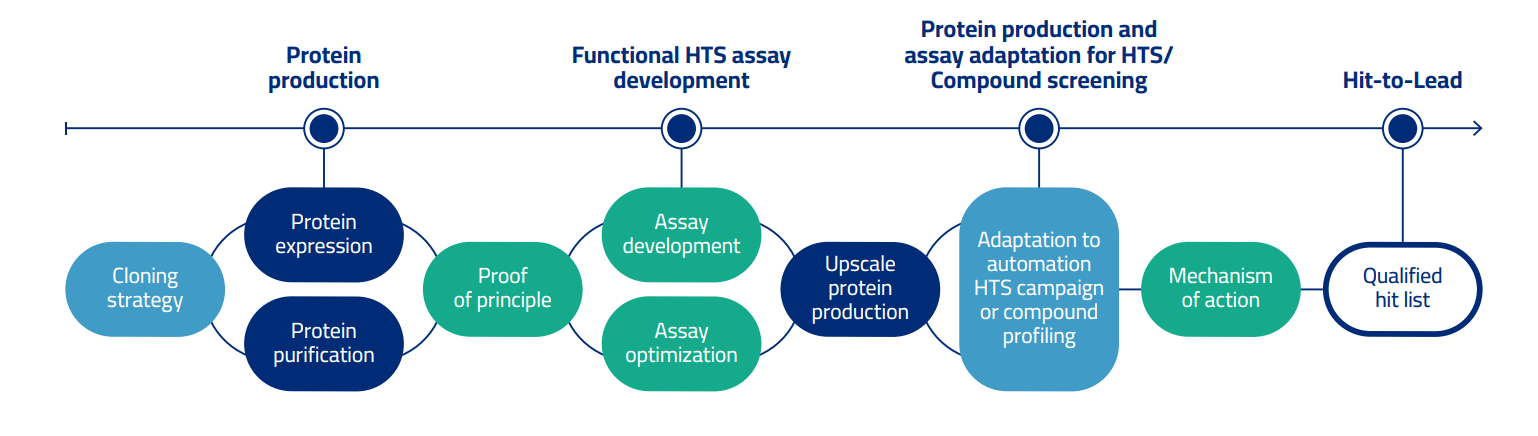
We offer a streamlined process from recombinant protein expression and purification, development of functional biochemical assays for High-Throughput Screening (HTS), and configuration of an array of secondary assays to proficiently assist Hit Identification, Hit-to-Lead and Lead Optimization.
Protein
production
Functional HTS assay
development
Protein production and
assay adaptation for HTS/
compound screening
Hit-to-Lead

Cell-free assays are configured in 384 or 1536 format, while assays transferred by clients can be miniaturized from any previous format. Readouts cover HTS-compatible state-of-the-art screening technologies such as:
- Fluorescence, including:
- Fluorescence intensity
- TRF
- FRET
- TR-FRET
- Fluorescence polarization - Absorbance
- Luminescence
- AlphaScreen®
- Radiometric systems, including:
- Flashplate technology
- Scintillation Proximity Assay (SPA)
Alternative assay types and different recombinant versions of the target are assessed in parallel applying a matrix approach during the initial proof of principle phase, which allows that the assay combination displaying the highest performance is identified and shortlisted for HTS, thus ensuring a higher effectiveness of the downstream drug discovery program.
The expertise of our team for the configuration of enzymatic assays for HTS covers the full range of target classes, such as:
- Oxidoreductases, including:
- Histone demethylases
- Dioxygenases
- Metabolic enzymes (Warburg effect) - Transferases, including:
- Protein kinases (Ser, Thr, Tyr)
- Nucleotide kinases
- Metabolic enzymes (Warburg effect) - Lyases, including:
- Metabolic enzymes (Warburg effect) - Hydrolases, including:
- Peptidases (Asp, Cys, Ser, Met, Thr)
- Lipases
- HDACs
- Phosphatases
- DNA/RNA/Nucleotide hydrolases
- Phosphodiesterases
- Deubiquitinases - Isomerases
- Ligases
In-house assembled libraries of surrogate substrates for protein kinases, proteases and esterases can be screened to explore enzyme selectivity and to accomplish HTS using a target-specific substrate, a key element to improve the overall quality of the outcome of hit discovery programs.
Enzymatic assays for HTS are in-depth characterized and optimized, with determination of their kinetic parameters, gauge of the stability of key assay components, validation with tool reagents and reference compounds, with assessment of their robustness for compound testing when performed under workstation-assisted conditions.
Learn more about functional enzymology
Cutting-edge drug targets are represented by interacting biomolecules, and we are proficient in assisting the development of any kind of cell-free interaction assays for compound screening. They include assays monitoring both binary interactions, as well as ternary and higher-order complexes.
In more detail, biochemical proximity assays are typically developed using TR-FRET or Fluorescence Polarization readout systems, and a non-comprehensive collection of case studies developed at Axxam comprises:
- Protein-protein interaction
- Protein-peptide interaction
- Protein-DNA interaction
- Protein-RNA interaction
- RNA-targeting compounds
- Protein-compound interaction e.g., PROTACs, molecular glues
Our proximity assays for HTS have assisted successful drug discovery programs aimed at modulating cellular protein/RNA dynamics seeking for Proteolysis-Targeting Chimeras (PROTAC) or Ribonuclease Targeting Chimera (RIBOTAC), protein/RNA stabilizers or molecular glues aimed at restoring or enhancing physiological cellular turnover of the target.
Proximity assays undergo a thorough assay optimization to effectively meet the criteria to access HTS, with determination of their affinity parameters, stability of interacting components, validation with tool reagents and reference compounds, with assessment of their robustness for compound testing when performed under workstation-assisted conditions.
A key milestone in drug discovery programs is the achievement of the unequivocal proof that a molecule establishes direct interaction with the target.
We offer a platform for investigating compound-target interaction in solution, with no requirement for target immobilization through Thermal Shift Assay/Differential Scanning Fluorimetry (TSA/DSF) by measuring ligand-induced modulation of target thermal stability. TSA/DSF is performed under HTS-compatible 384 format with systems integrated into an automated screening station, and data analysis is assisted using dedicated software which ensure a throughput comparable to standard HTS.
Moreover, an in-house devised dye library and a platform for assay optimization are available to be screened for those proteins which turn out to be hardly tractable under standard experimental conditions. Remarkably, TSA/DSF platform can be proficiently applied to characterize and validate not only ligands binding to proteins, but also compounds targeting nucleic acids, such as RNA.
The TSA/DSF platform is complemented by Microscale Thermophoresis (MST) and Temperature Related Intensity Change (TRIC), two biophysical technologies that enable the precise determination of protein-ligand binding affinities in solution, which can be performed under mid-throughput conditions to characterize compound series or to efficiently screen targeted libraries and fragment collections.
