Target Identification and Validation
Effective drug discovery starts with the strategic selection of relevant and actionable biological targets. At Axxam, we support pharmaceutical and biotech companies throughout this critical early phase by combining state-of-the-art technologies, artificial intelligence (AI)-driven insights, and deep biological expertise.
Target identification
Many of today’s most promising drug discovery paradigms are data-driven and begin with an evidence-based foundation, where potential targets are validated against clinical data from the earliest stages. Modern target identification transcends traditional approaches by leveraging AI-enabled analytics and sophisticated biomedical knowledge bases built on the intersection of expansive biological data and clinical insights. This approach helps uncover therapeutic opportunities that might otherwise remain hidden.
The strength of this methodology lies in harmonizing diverse data streams—genomic sequences, proteomic analyses, clinical trial results, and real-world evidence—into a unified knowledge ecosystem, providing context and connectivity. Advanced algorithms and specialized deep analytics are well-placed to interpret these complex relationships, illuminating novel therapeutic pathways grounded in clinical relevance and opening new frontiers.
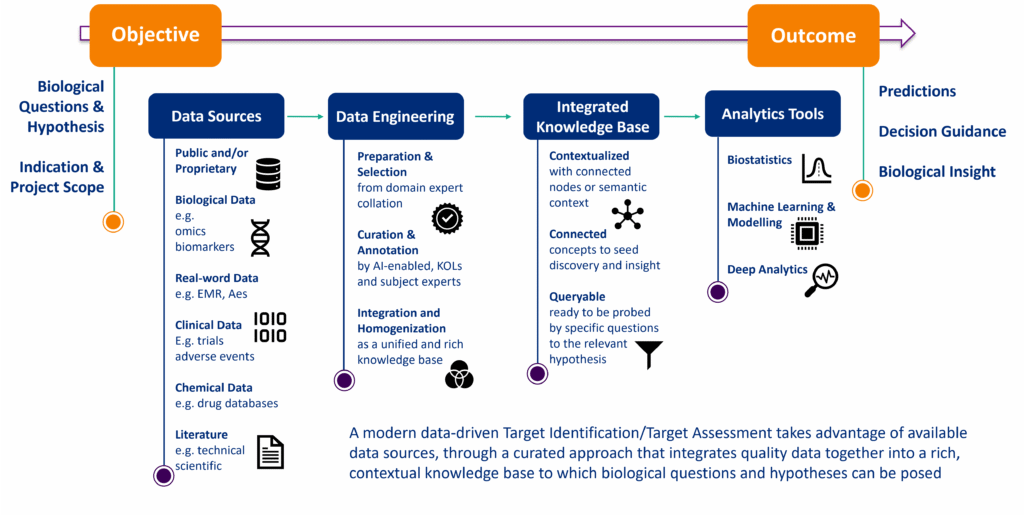
By integrating this evidence within interconnected knowledge networks, analytics can begin to trace biological pathways from mechanisms of action to patient impact providing insights with greater confidence. This integrated approach helps reduce downstream development risks by stacking the deck in favor of targets likely to demonstrate clinical relevance before substantial investment is made.
At Axxam, we collaborate closely with industry leaders in biomedical deep analytics and artificial intelligence, such as Molecular Health GmbH, to support the target identification process: we apply in silico strategies – such as integrating clinical and omics data to prioritize targets – and translate these insights into actionable outcomes through wet-lab validation. These AI-driven target identification strategies can seamlessly transition into our experimental workflows, including assay development and fully integrated early drug discovery programs from target to lead.
Target validation
Once promising targets are identified, the next essential step is validation – demonstrating the direct involvement of a molecular target in a disease mechanism and providing evidence that its modulation is likely to have a therapeutic effect.
Axxam provides a comprehensive portfolio of complementary techniques to build confidence and reduce the risk of late-stage failures.
1. Genetic approaches
Genetic modulation and overexpression studies help establish a target’s role in disease mechanisms. By altering gene function in relevant cellular models, such as human induced pluripotent stem cells (iPSCs), primary cells and/or immortalized cell lines, we can validate biological impact in a controlled, disease-relevant setting. The following approaches support AI-driven target identification by linking target modulation to measurable cellular outcomes:
- Knock-out (KO) via CRISPR/Cas9
- Knock-down (KD) using CRISPR-i, siRNAs, shRNAs, or antisense oligonucleotides (ASOs)
- Overexpression via transfection, transduction or CRISPR-a approaches
2. Expression profiling
Expression profiling assesses the presence and distribution of the target in healthy versus diseased tissues. This approach helps determine whether a potential drug target is expressed in relevant tissues and whether its expression correlates with disease progression or severity.
More specifically, expression profiling assesses:
- Differential expression: Changes in expression levels that may indicate the target’s involvement in disease mechanisms, for example, overexpression in tumors compared to normal tissue.
- Target expression across disease models: Correlation of target expression levels in different cellular disease models, supporting its relevance as a therapeutic target.
This evidence is key to de-risking decisions and supporting early validation strategies.
3. Functional assays
Functional assays measure the biological activity of the target and the effects of modulating it, often using tool molecules to demonstrate desired outcomes in vitro.
Biochemical assays are typically cell-free and focus on molecular interactions and enzymatic activity, providing critical data about the biochemical function of targets. These assays are essential early steps in drug discovery for evaluating molecular interactions and biological activity under controlled conditions.
These assays evaluate the effects of drugs or compounds on biological targets and can determine the potency, efficacy, and mechanism of action of the molecules. They are crucial in drug discovery to identify promising therapeutic candidates and to understand their action at the molecular or cellular level.
These types of assays investigate the effects of compounds on specific cellular signaling pathways and biological processes within live cells. By using cell-based assays, it is possible to measure cellular responses such as proliferation, apoptosis, gene expression, and receptor-ligand interactions. These approaches provide physiologically relevant contexts to study complex biological interactions and validate drug targets by confirming that modulating the target produces the expected cellular outcome.
These diverse formats allow Axxam to validate whether modulating a target produces the expected outcome – further strengthening AI-driven target identification and validation efforts.
4. Phenotypic analysis
Phenotypic assays aim to understand the biological impact of potential drug targets by observing measurable changes in cellular behavior or molecular profiles. These high-content methods offer a rich layer of validation data:
A high-content imaging technique that uses multiplexed fluorescent dyes to capture morphological changes in cells. It provides a rich phenotypic fingerprint, useful for comparing drug-induced effects and inferring target engagement or off-target effects.

Multi-electrode array (MEA) platforms measure electrical activity or calcium dynamics in neurons or other excitable cells. Changes in calcium oscillation patterns upon target perturbation reflect functional phenotypic outcomes, especially relevant in neuroscience and cardiotoxicity studies.
This involves profiling gene expression changes (e.g., via RNA-seq) after target modulation. It helps identify downstream pathways influenced by a target and supports mechanistic understanding of phenotypic effects. Transcriptomic services are provided in collaboration with trusted partners.
Mass spectrometry-based analysis of global or targeted protein changes in response to perturbations. It allows assessment of changes in protein abundance, modifications, and interactions, contributing to the validation of target-specific effects. Proteomic services are provided in collaboration with trusted partners.
Together, these complementary approaches provide a multi-dimensional view of target function and compound activity, enhancing confidence in target validation.
MEA and calcium oscillations
Activity map (left) and spike sorter with respective raster plot showing acitivity recorded from iCell Motor Neurons (FCDI) cocultured with iCell Astrocytes (FCDI) at 24 DIV
Why choose Axxam for validating AI-driven insights?
By integrating advanced biological platforms with insights from AI and in silico modeling, Axxam offers a complete solution for AI-driven target identification and validation. Our scientific teams collaborate closely with strategic partners to move from digital prediction to wet-lab confirmation with precision, speed, and confidence.
Whether you are prioritizing early-stage targets or preparing for lead optimization, Axxam provides the expertise, tools, and flexibility to help you advance the right target – right from the start.


