Organellar Electrophysiology
Over 80% of transport processes due to ion channels and transporters are localized inside the cells where they play a crucial role in maintaining ion homeostasis and signaling, thus regulating several physiological processes such as apoptosis and lysosomal degradation.
Organellar ion channels and transporters are emerging as key disease modulators and promising targets for the treatment of cancer, pain, neurological disorders, and aging. There is a need to overcome the challenges associated with studying these targets at organellar level and increase the degree of resolution when characterizing new drug candidates.
Your channel, our solution
As a unique addition to our electrophysiology platform, we have implemented and developed cutting-edge techniques to allow the functional and pharmacological study of electrogenic transporters and ion channels directly at organelle level.
Driven by the desire to translate our clients’ needs into innovative solutions, our expert scientific team overcame technical challenges to establish NucleoPatch, LysoPatch and MitoPatch assays aiming to study electrogenic proteins in their native environment (nuclei, lysosomes, and mitochondria, respectively), and pioneering new tools for drug discovery industry.
Our clients can take advantage of our exclusive expertise in recording ion channel activity not only by manual patch clamp, but also in automated patch clamp in 384 format (available for LysoPatch), on intact organelles to perform a comprehensive biophysical and pharmacological characterization to identify hit compounds.
Discover below our capabilities in a nutshell
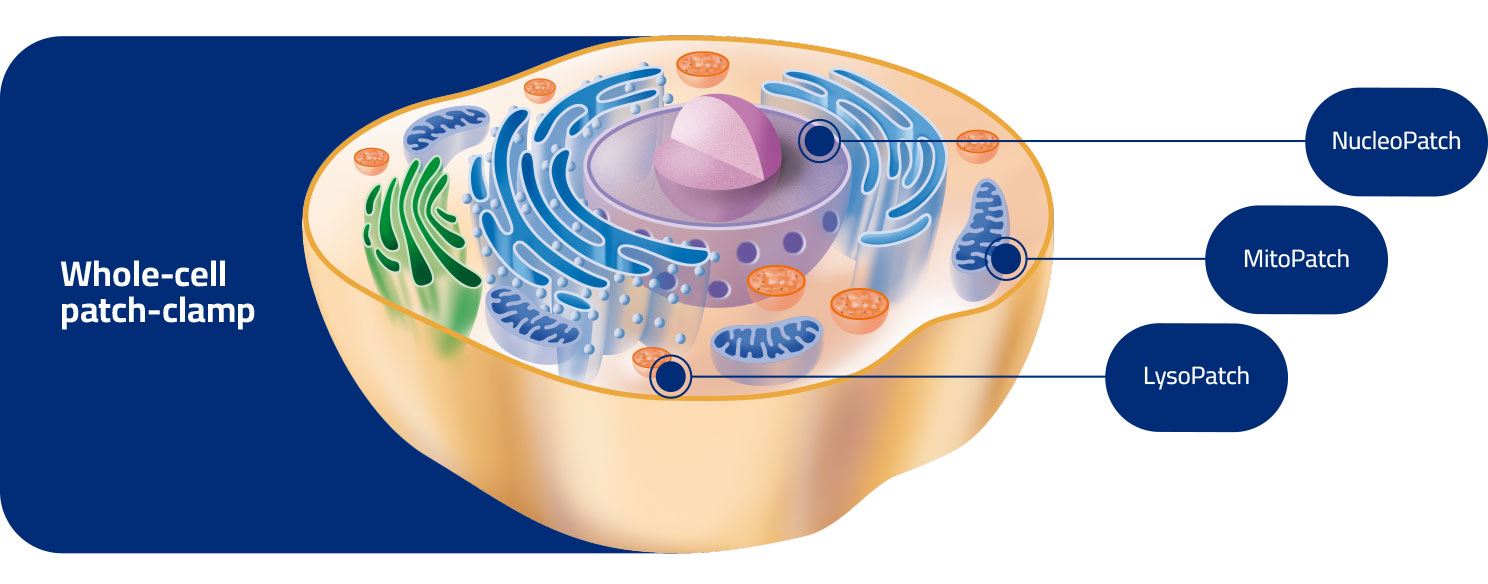
Studying ion channels endogenously expressed on the outer membrane of isolated nuclei.
- Manual patch-clamp
- “Nucleus attached” recordings
- Case study: Single channel characterization (calcium dependency, pharmacological validation, compound profiling) of ryanodine receptor (link to the 2020 BPS poster)
Patch-clamp on the inner membrane of isolated mitochondria.
- Manual patch-clamp, planar patch-clamp (Port-a-Patch, Nanion)
- “Whole-Mito”, “Mito-Attached” (single channel) and Inside-out (single channel)
- Biophysical and pharmacological characterization (single-point or cumulative dose-response experiments) of the target of interest
- Manual and automated patch-clamp (SyncroPatch 384, Nanion)
- “Whole-Lyso” (manual and automated patch-clamp), “Lyso-attached” (single channel) and inside-out recordings (single channel) (manual patch-clamp)
- Biophysical and pharmacological characterization (single-point or cumulative dose-response experiments) of the target of interest
- Compound profiling & mode-of-action studies (i.e., compound effect at different pH with possible change of luminal environment)
We don’t stop innovating!
We are currently working on:
- Increasing throughput by ad hoc adaptation of SP384 protocol to enable work on isolated mitochondria.
- Assay development and adaptation of manual patch-clamp technique to record ion channels expressed on lysosomes isolated from iPSC-Derived cells.

