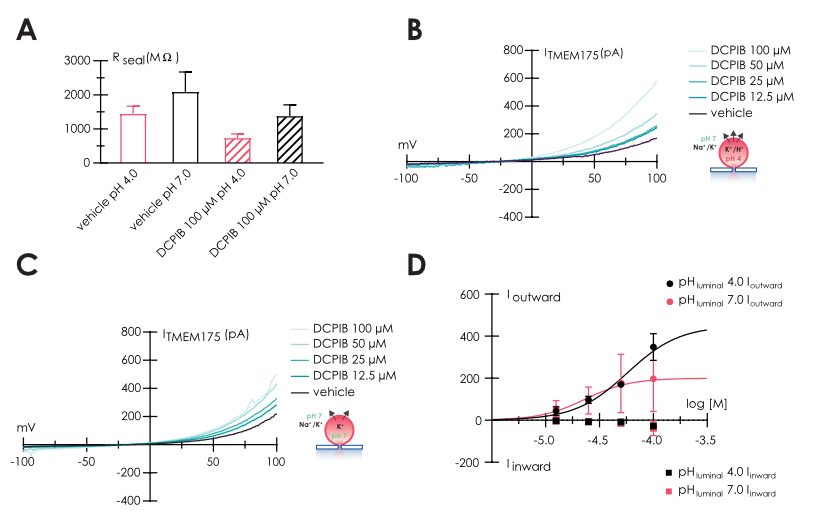
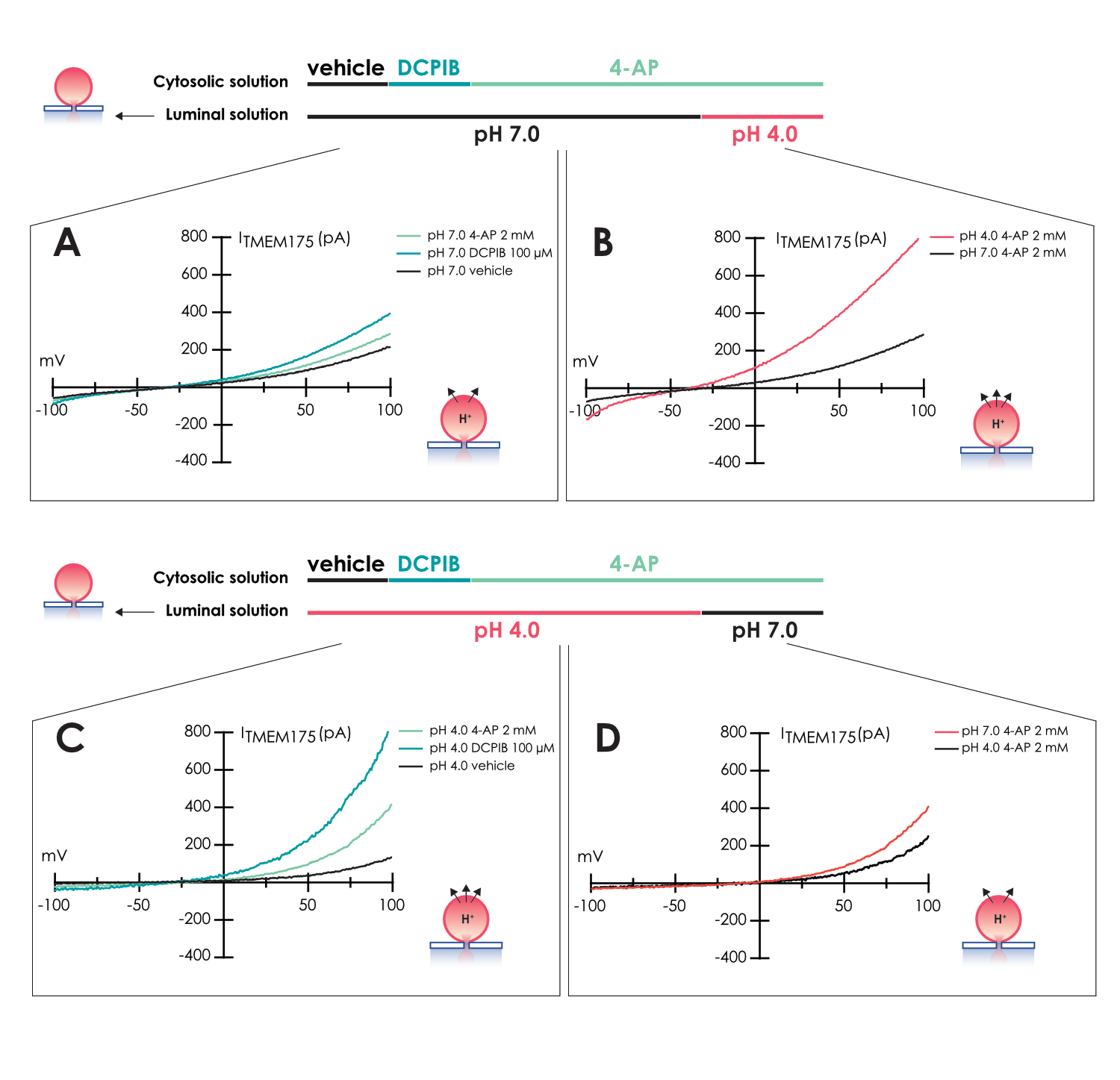
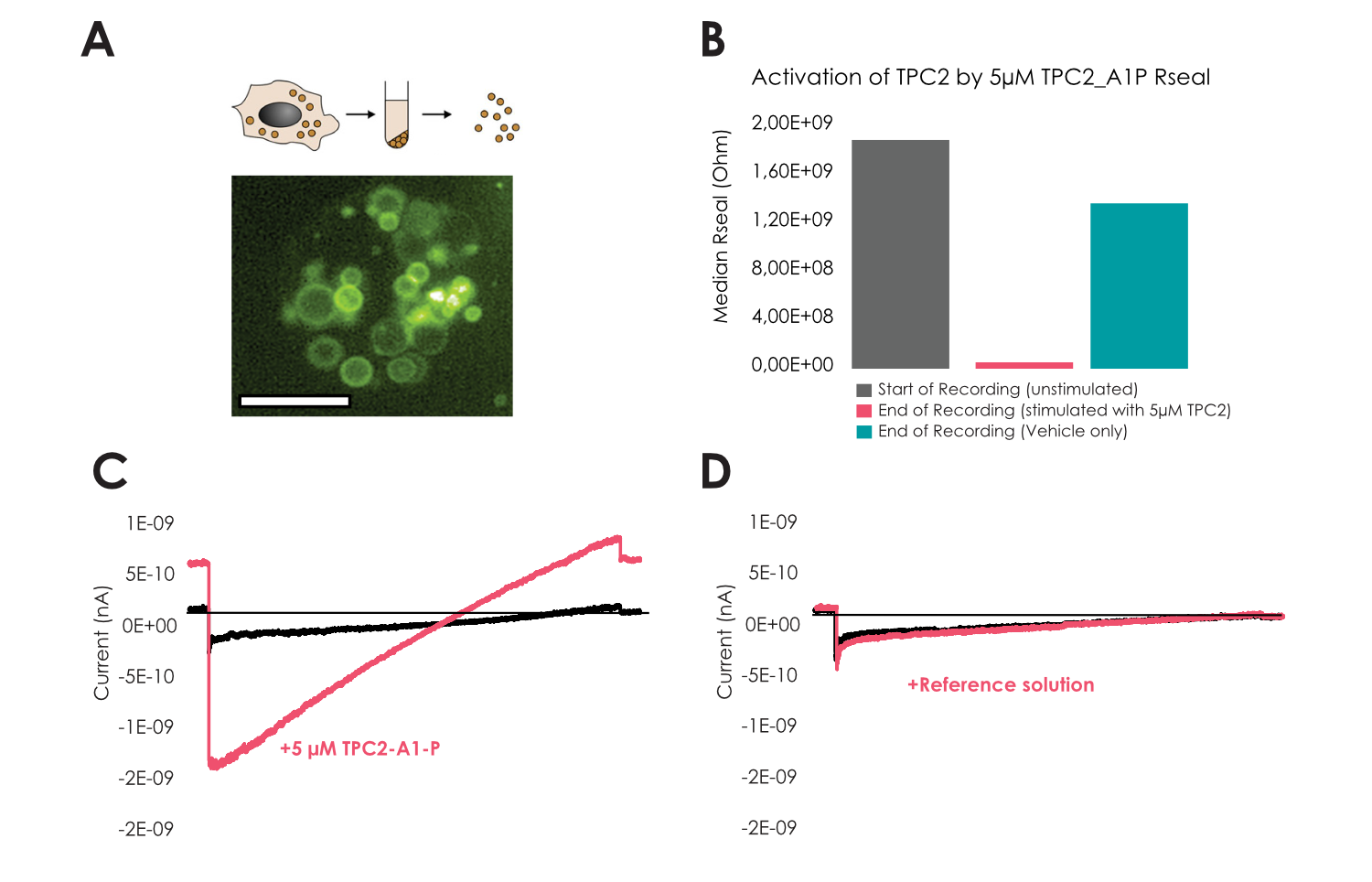
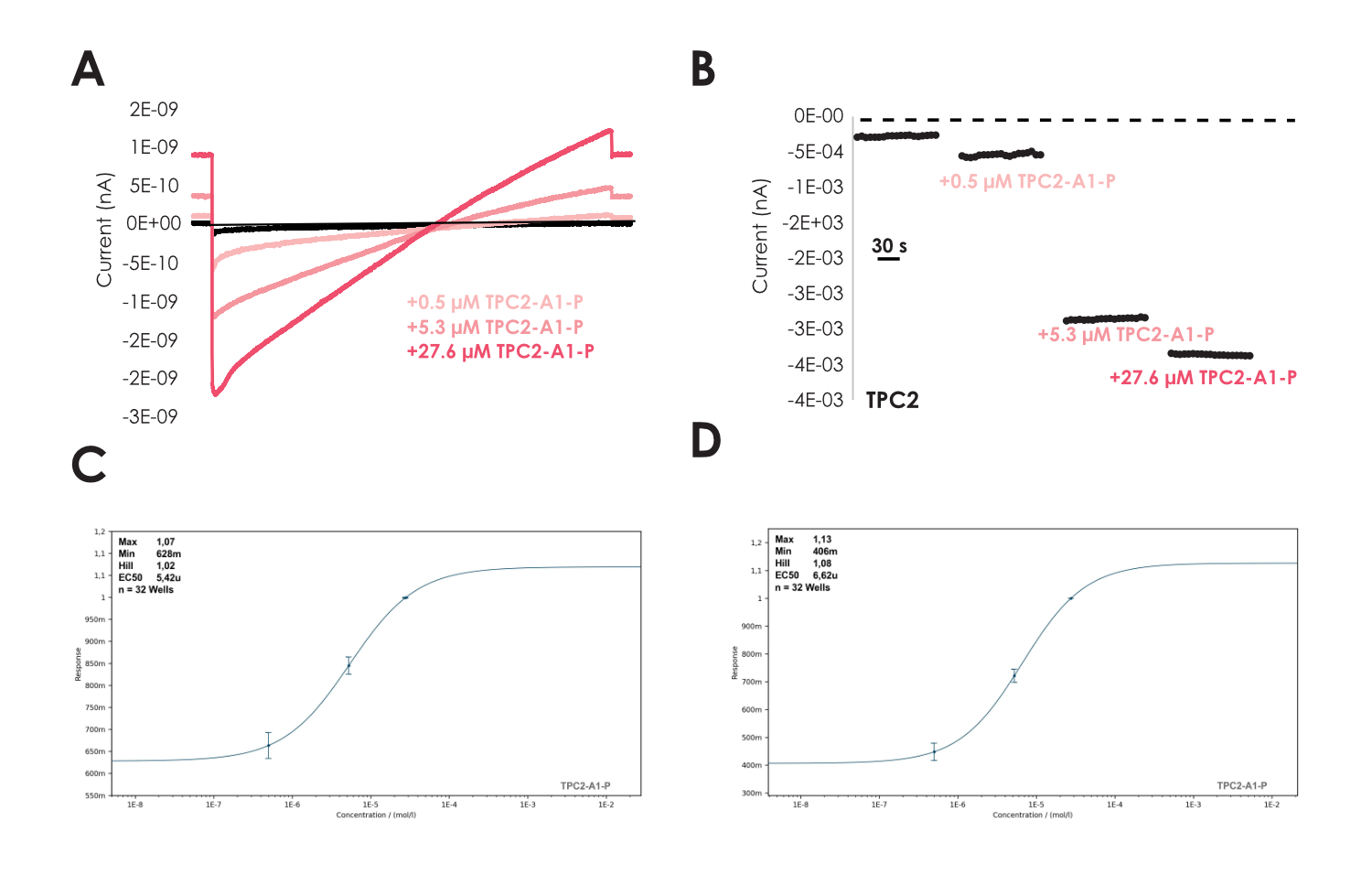
Figure 1
A – Isolated lysosomes stained with LysoTracker™ Red DND-99 (Invitrogen); images at different magnifications were acquired using the Operetta (Perkin Elmer).
B – Average diameter calculated at different dilutions: 3.0 ± 2.1 µm (1:10); 2.0 ± 1.3 µm (1:20); 2.4 ± 1.7 µm (1:50); data are presented as mean ± SD.