High-Throughput Screening
At the core of the drug discovery process is the screening of hundreds of thousands of compounds in a high-throughput manner using in-vitro assays. Time-sensitive and cost-effective strategies are crucial for the identification of valuable hits with a specific pharmacological activity, such as those modulating a target or a cellular pathway of interest.
At Axxam, we support our clients during the early phases of the drug discovery process, from assay design to the performance of High-Throughput Screening (HTS) campaigns, and even further during the Hit-to-Lead process. Our expertise enables us to develop the most suitable hit-identification strategy, ensuring a reliable compound class that can be pursued for further drug development.
We not only find the needles, but also sort the haystack!

The fundamental ingredients for a successful hit discovery program are meaningful assays, high quality compound libraries and well planned screening campaigns. To run an HTS campaign, clients can access our assay development services, select one of our ready-to-use assays or transfer their own validated assays to us. We carry out hit-discovery programs using either compound collections available at Axxam or our clients’ preferred libraries, which are handled securely at our facility. Besides small molecules, we also screen chemical mixtures, natural product extracts, oligonucleotides, and antibodies. The most appropriate screening strategy is selected for any screening campaign according to the specific target’s characteristics. This approach ensures the highest probability for a successful HTS with meaningful results, providing the foundation to generate innovative lead compounds.
HTS platforms & technologies
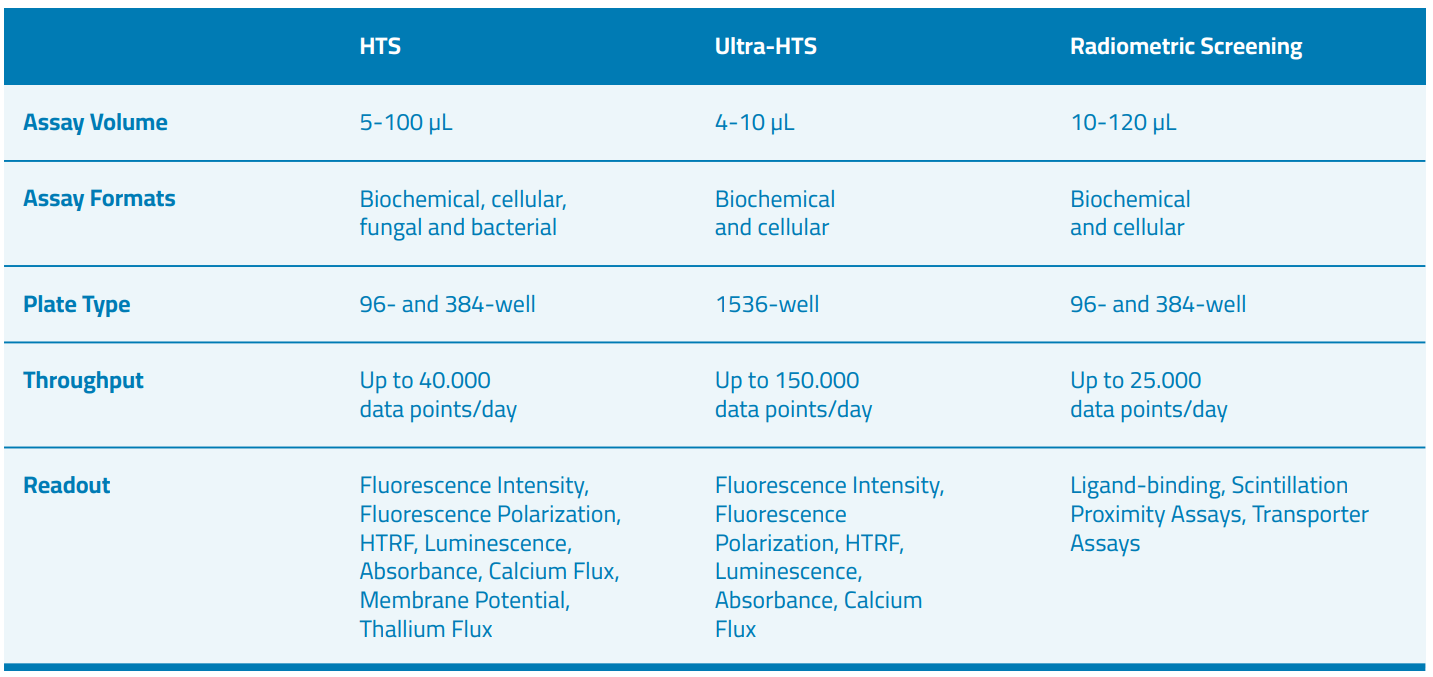
We provide access to fully automated, state-of-the-art screening platforms designed to run cell-free and cell-based assays in a miniaturized format using a variety of detection technologies such as luminescence, fluorescence (intensity polarization, time-resolved such as HTRF or LANCE, calcium flux, membrane potential, thallium flux), absorbance, as well as radiometric measurement using ligand-binding, scintillation proximity assays (SPA), flashplate assays and transporter assays.
Cell-based screenings can also be performed using electrophysiological, quantitative genetic expression, and phenotypic imaging-based readouts.
We run automated screening campaigns with either cell-free or cell-based assays (homogeneous and non-homogeneous) in both high-throughput screening (384 well plate) and ultra-high-throughput screening (1536 well plate) formats:
For projects targeting ion channels or electrogenic proteins, our clients can rely on high quality patch-clamp screenings run on the SyncroPatch instrument (Nanion) integrated in a fully automated robotic platform. As well as primary screenings on a large compound collection, this instrumentation can be used for:
- Clone selection
- Compound profiling
- Hit validation/SAR studies
We can run gene expression quantification analysis in a medium/high-throughput manner based on TaqManTM RT-qPCR assays with several advantages:
- Suitable for any cell type or target gene
- More “natural” conditions
- Multiplexing – more genes can be easily investigated
- No false-positive hits caused by readout interference
- Toxic compound/plate effect revealed by HK analysis
Studying the effect of bioactive compounds on biological pathways in a complex cellular environment requires both, a highly sensitive instrument for imaging acquisition in miniaturized format (such as the Operetta® and Opera Phenix® systems from PerkinElmer), and the experience of a skilled team capable of analyzing the huge amount of information obtained from phenotypic screening.
- Immortalized cell lines, iPSC-derived cells and primary cells
- Cell painting approach
Hit validation
The hit validation phase stands as a pivotal stage following a screening campaign, holding immense significance in the drug discovery process. This phase rigorously evaluates and validates the initial hits identified during the screening stage. Its importance lies in the confirmation of the biological activity, specificity, and mechanism of action (MoA) of the hits identified in the screening campaign.
Hence, the availability of a panel of secondary assays (specificity, selectivity, orthogonal, MoA) tailored towards the specific needs of individual projects, is critical to generate the relevant data. Beyond ensuring the target specificity and desired MoA of a hit compound, the hit selection process is guided by additional medicinal chemistry considerations. These include a check of the compound purity and re-test from solid material. Information on analogs, also from outside commercial sources, might provide initial clues on emerging structure-activity relationships. Finally, few simple in vitro assays to probe basic ADME (e.g. protein binding, plasma stability) and/or physchem properties (e.g. lipophilicity) will help to focus early on hit compounds combining a specific bioactivity with desirable secondary properties for further optimization.
Based on our extensive experience, we will support our clients in designing a screening cascade tailored to the individual target and project needs to ultimately ensure a rigorous hit validation and focus downstream investments on hits more likely to progress.
